Innovation
Expertise and know-how in advanced
wound care gelling fibre technologies
Supporting clients from concept development to market launch
In the words of our R&D Director “We come to work every day because we are passionate about what we do”.
At SFM we invest a large amount of our resources in our gelling fibres development (R&D) activities. Our R&D Team makes a real contribution towards our marketing activities and the development of new products across our business, and for our customers.
Sustainability
R&D at SFM has a sustainable mindset. Hence, much of our product development ethics are focused on using sustainable materials where possible. From our Alginates’ fibres through to our modified cellulose portfolio. We collaborate across the business with our marketing colleagues and production engineers, in developing sustainable production processes.
In addition, SFM has also developed phthalate free barium sulphate x-ray detectable monofilaments (yarn), known as Micropake®. Our x-ray detectable yarn is used in surgical sponges and other gauzes / swabs for surgical purpose, with applications ranging well beyond the medical industry. SFM is one of the very few European manufacturer of x-ray detectable yarn. Our team has expertise in varying the strength, diameter and properties inherent to these yarns.
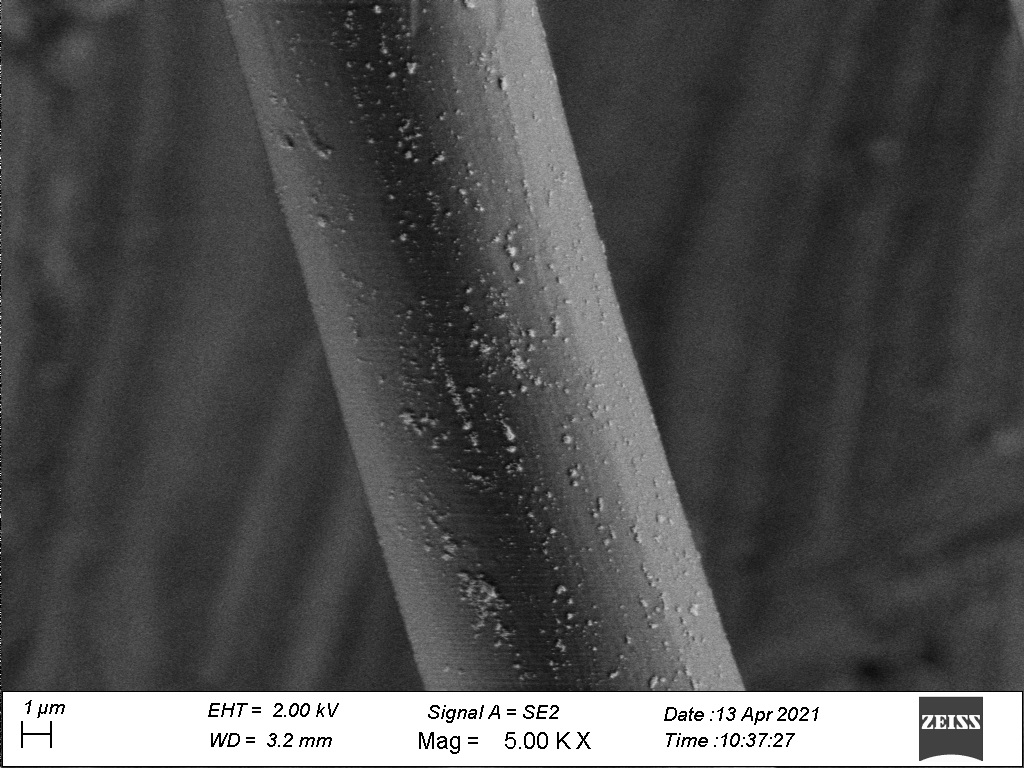
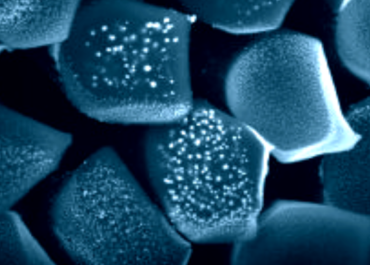
Nanoparticles
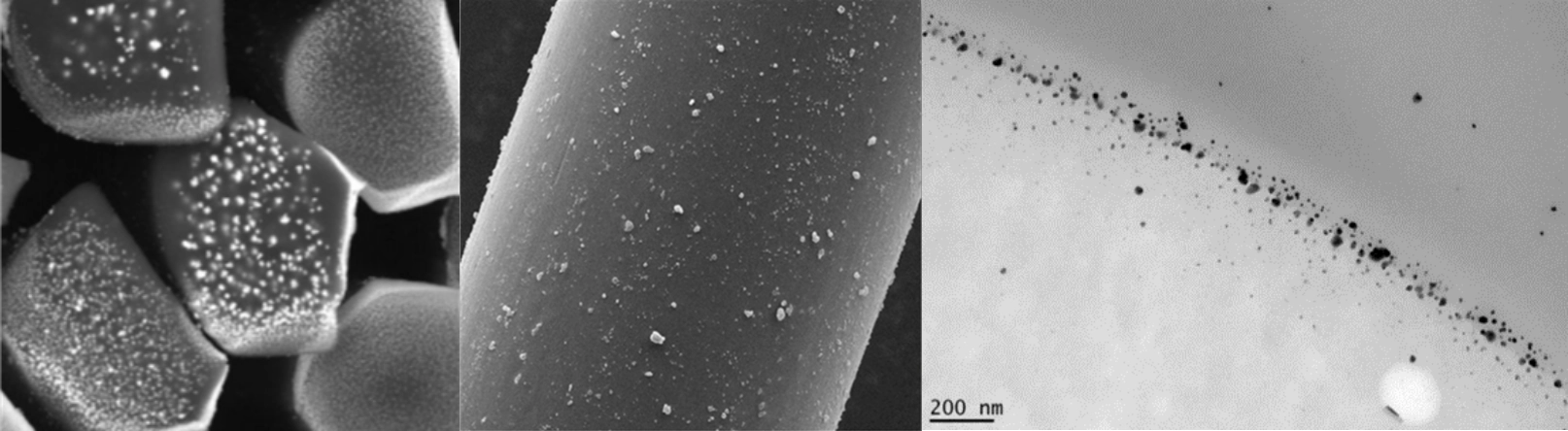
Our R&D scientists have specific experience in nanoparticle technologies. SFM has developed a portfolio of antibacterial nanoparticles, specifically applied to our alginates and modified cellulose fibres. Furthermore, the R&D team can adapt the chemical and physical properties of our fibre products to meet our customers’ unique requirements.
Whether those needs are:
- Antibacterial product development and testing
- Bespoke non-woven fabric development
- Filled thermoplastic yarn development
- Functional chemically converted fibre development
- Radiopaque yarn development
- Small scale fibre and non-woven fabric production for pivotal studies and regulatory submissions
- Treatment of fibres with active agents
- Antibacterial product development and testing
SFM has in-house research and quality control laboratories, specifically used to test our products’ quality and physical properties. From gelling fibres development properties, tensile strength wet and dry, absorbency through to diameter. In addition, SFM houses full scale-up capabilities, which provides our partners with the ability to scale up their laboratory conceptual technologies.
Follow us on LinkedIn for our companies latest update.
Our Team
SFM has an experienced team of PhD Biomaterial, Biology, Biochemistry scientists. This ensures that our products stay at the forefront of the latest technologies. SFM works on several projects in partnerships with its customers, Universities as well as developing its own portfolio of IP. In addition, our R&D team collaborates with several key opinion leaders, clinicians and health professionals in order to understand the key products’ characteristics required for better clinical outcomes. SFM has a legacy spanning 90 years in fibre spinning and non-woven fabric manufacturing.
Our R&D team’s experience is not limited to developing the latest technologies, but encompasses a strong manufacturing know-how, which in our view is unparalleled in our industry.

Dr Graeme Kettlewell
R&D Director

Dr Gareth Wynn-Jones
R&D Manager

Dr Ewa King
Senior Research Scientist

Steve Homer
Research Technician

Klaudia Piorunowicz
Laboratory Technician
FREQUENTLY ASKED QUESTIONS
If you need some help, we have a list of frequently asked questions and answers. Click here to contact us.
With a modern card and needle loom located in a Class 8 clean room SFM have enviable pilot plant facilities. Our capabilities range from making non-woven fabrics from a small amount of fibres to material for stability and other pivotal studies. With unparalleled experience in non-woven fabric manufacture, SFM have the ability to add together multiple layers of fabric such as scrims to improve strength, antimicrobial layers, activated charcoal to capture and to blend together fibres with different properties to produce non-woven fabrics with unique properties tailored to your requirements.
SFM have recently added an ultrasonic spray line to enhance the ability to functionalise wound dressings and fabrics. With this capability installed it is possible to coat fabrics with a vast array of additives to provide new performance benefits.
Using our pilot scale multi filament wet spinning line, the R&D team at SFM can develop fibre concepts on a scale suitable for proof of concept studies. This has been used by SFM and by customers to investigate the feasibility or performance of fibres and non-woven fabrics on a small scale before committing to expensive large scale trials or equipment modifications. The equipment has been recently used to investigate blending polymers to produce a co-spun fibre, understanding the effect of processing parameters on fibre and fabric performance and how residual polymers, processing aids and cleaning fluids interact with each other before plant trials take place.
SFM Pilot facilities include
- Automatex card and cross folder
- TecTex needle loom
- Multifilament wet spinning
- Multifilament melt spinning
- Ultrasonic spray line
- Chemical conversion of fibres
- Fibre coating and treatment
Our R&D department can offer a broad range of developments depending on your exact needs. Recent customer projects successfully managed by our R&D team have included:
- Delivering to a hospital based research team successful prototypes of a dressing for the management of an extremely rare skin condition
- Development of prototypes of feminine hygiene products
- Different variants of our long standing Micropake radiopaque yarn for medical and non-medical applications
- Researching the prior art and literature to develop and patent a novel gelling forming fibre dressing to allow our customers to challenge the market leader
- Spinning fibres, blending with a second fibre and providing a non-woven dressing in bulk for the customer to cut, package, sterilise and sell under their own regulatory approvals
- The chemical modification of fibres for a customer to process further using their own equipment
- The development of bespoke fibres for a wound care application for a customer to process further using their own equipment
- The development of novel nanoparticle impregnated fibres and the scale up of the manufacturing processes for fibres and fabrics for the customer to sell using SFM’s regulatory approvals
Collaborating with our manufacturing and regulatory teams positions SFM uniquely to offer customers in the advanced wound care market development spanning the range of
- Bespoke fibres for sale as tow or staple fibre
- Bulk non-sterile non-woven fabric for sale as wound dressings
- Fibres and fabric for non-medical applications
- Finished packed and sterile wound dressings with regulatory clearances
Our R&D team innovate in test method development to provide the evidence needed to support product development, regulatory filings, marketing claims or scientific literature. With a large database of product data SFM can identify how a concept performs and where it fits into the market.
SFM have a wide range of analytical techniques available to support your research and development project. Our laboratory is fully equipped for testing wound dressings and includes elemental analysis for measuring the silver content and trace elements. We also have the capability to perform scanning transmission electron microscope (STEM) and atomic force microscopy to obtain detailed images of the surfaces of fibres. Antimicrobial and antibiofilm performance is a vital part of the clinician’s armoury in managing chronic wounds. SFM can perform a wide range of testing to understand how your antimicrobial agent performs from minimum bactericidal concentration (MBC) studies using robotic high throughput screening, zone of inhibition and shake flask to the US FDA’s preferred modified AATCC-100 method.
SFM have a regulatory team that is second to none and, working with the R&D team, we can offer a full range of testing to support your product development from concept to market. In the recent past we have supported customers with:
- Antimicrobial performance evaluation
- Biocompatibility testing
- Chemical characterization
- Materials characterization
- Performance testing
Although wound care and healthcare is our main focus, SFM’s unique heritage and rich skillset enable us to develop fibres and fabrics for many industries.
SFM have experience of working with a wide range of fibres including
- Alginates
- Cellulosic, nylon and polyester scrims
- Fibres from animal derived materials
- Filled thermoplastic yarns
- Gelling fibres
- Metallic coated fibres and fabrics
- Polysaccharides
- Superabsorbent fibres and fabrics
- Synthetic fibres